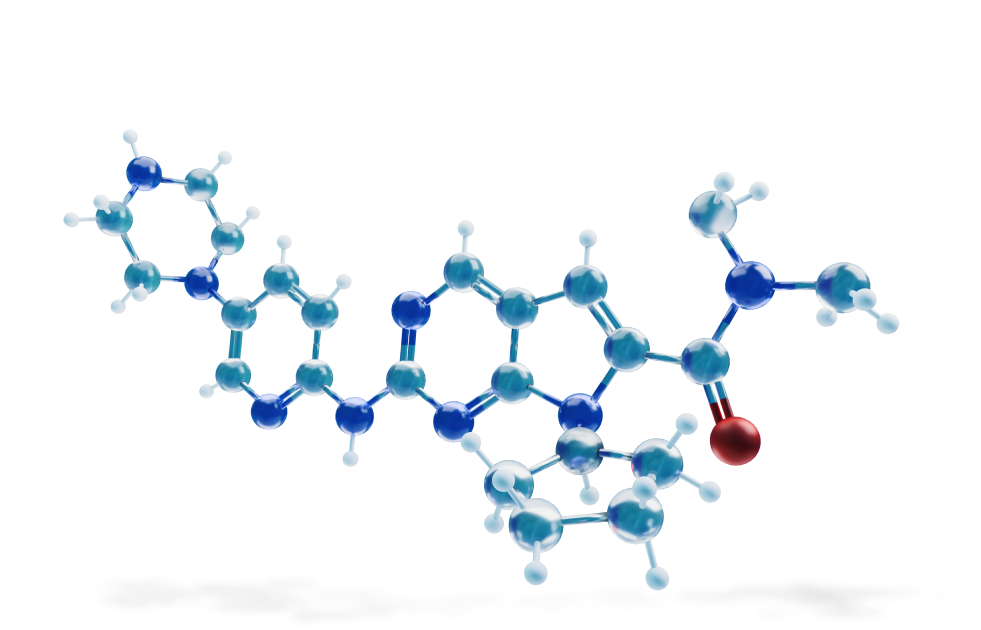
INHIBITOR OF CYCLIN-DEPENDENT KINASE (CDK) 4 AND 6
Treatment of postmenopausal women with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative advanced or metastatic breast cancer as initial endocrine-based therapy.
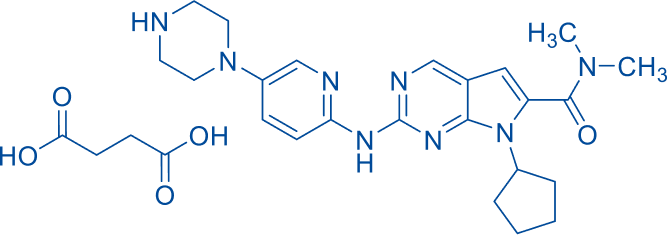
Polymorphic Form A (US9193732B2) confirmed.
GMP Production capabilities up to 500 kg/year.
Workshop OEB 4 with engineering control measures.
Chinese manufacturer freedom to operate for this product.
Development samples and standards available with CoA.
Impurities deeply studied. Total impurities around 0.1%.
Purity > 98%.
No monograph in force. In compliance with general regulatory requirements.
Stable room temperature.
Industrial quantities available in Q3 2025.
Competitive price based on market.






PATENT PROTECTION